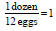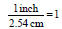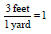What is Chemistry?
| Chapter 1 Chemistry: Matter and Measurement |
What is Chemistry? • On your note card, write your name at the top and numbers 1 - 4 down the left side. When each word is shown below, write the first thing that comes to mind. • 1) Chemistry • 2) Chemicals • 3) Science • 4) Experiments |
| Things You Should Know… • Names of rows in the Periodic Table (periods) and columns (groups) • Names of the 4 main groups • Names of the 3 general types of elements (metals, nonmetals, metalloids) • SI units: 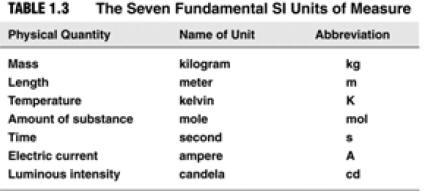 |
Things you should know… • Names and symbols of elements: • Numbers 1 – 38, Cs, Ba, Hg, Ag, Au, Sn, Pb • Zn2+, Cd2+, Ag1+ |
| Things you should know…. • Lab equipment  |
Measurements • Temperature  K, °C, °F K, °C, °F• C = 5/9 * (°F – 32) • Volume 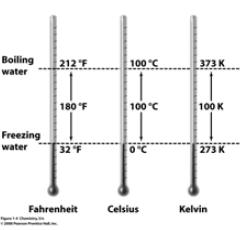 |
Elements and the Periodic Table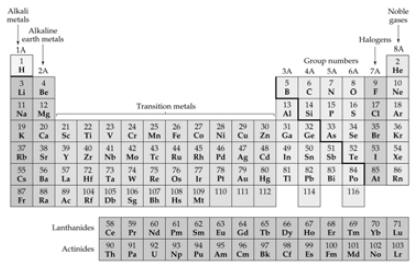 |
The Periodic Table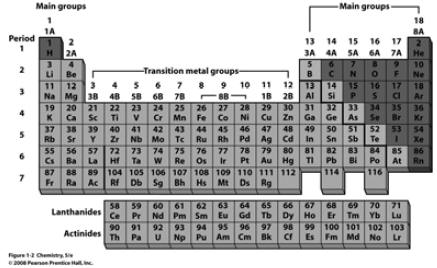 |
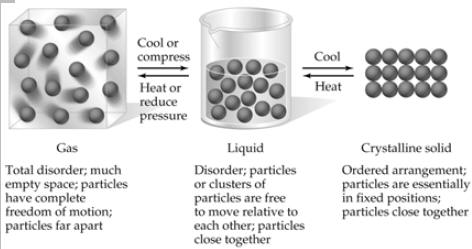
| States of Matter
|
Physical and Chemical Changes • What is happening below? 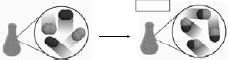 • Is this a physical or chemical change? • What’s the difference between the two? • Boiling water: physical or chemical? • Lighting a hydrogen |
| Physical or Chemical Change? • Do the following equations represent a physical or a chemical change? How can you tell? 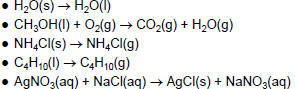 |
Density • D = m / v • Mass per volume • Diet versus regular coke • If a steel ball bearing weighs 54.2 grams and has a volume of 6.94 cm3, what is its density? • If a steel beam is measured to have a volume of 94390 cm3, how much does it weigh? |
| Density • Carbon dioxide gas is more dense than helium gas. Use the pictures below to explain why. 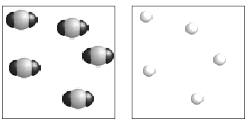 |
Scientific Notation • Use scientific notation to describe very large or very small numbers. • Negative exponents indicate small number, positive exponents indicate large number. • How can we describe the size of an atom? The size of the universe? • Scientific notation and powers of ten • Powers of Ten |
| Scientific Notation • Write the following numbers in scientific notation and place in order of increasing value: • 10, 0.001, 0.00002, 1 x 104, 1 x 10-3, 3 x 10-5, 8 x 105, 700000 • Add, multiply, and divide 3 x 103 and 2 x 102 |
How to Use Your Calculator • To enter 1.00 x 104 in your calculator, DO NOT enter “x” or “10”. • Instead, use the exponent key (“EXP” or “EE”). • Press: 1.00 “EXP” (or “EE”) 4 |
| Scientific Notation Practice • Add • Multiply •Divide • Adding and subtracting exponents is a good way to estimate answers to problems!! |
Scientific Notation Practice • Addition and Subtraction • Combine numbers with same exponent and add numbers • 7.4 x 103 + 2.1 x 103 = 9.5 x 103 • Multiplication • Add exponents and multiply numbers • 8.0 x 104 * 5.0 x 102 = 40 x 106 = 4.0 x 107 • Division • Subtract exponents and divide numbers • 6.9 x 107 / 3.0 x 10-5 = 6.9/3.0 x 107-(-5) = 2.3 x 1012 |
| Uncertainty in Measurement • What is the difference between precision and accuracy? What is shown in each picture below?
|
Uncertainty in Measurement • The true temperature outside is 71.2oF. Several thermometers made by one manufacturer record the temperature as 67.8, 68.2, 67.2, 67.6, and 68.0oF. • How would you describe this data in terms of accuracy and precision? Why? |
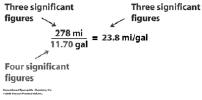
| Significant Figures • What are significant figures? • Why are they important? • When do you need to worry about them? • How many decimal places can you use on the rulers below (shown in cm)? 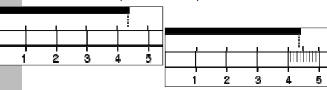 |
Significant Figures • Measurements versus calculations: device. 1 decimal place past the smallest increment. numbers reported. calculations. |
| Sig Figs • Measuring volumes  |
Determining Significant Figures • All non-zero digits are significant (335 cm). • Zeroes in the middle of a number are significant (3406 mg). • Zeroes at the beginning of a number are NOT significant (0.000345 km). • Zeroes at the end of a number and after the decimal point are significant (43.21000 g). • Zeroes at the end of a number and before the decimal point may or may not be significant (5280 ft). You will have to look at the measurement to determine this. |
| Determining Significant Figures • How many significant figures are in the following? •1.45 • 0 38 •0.0670 • 301.9 • 072.8 • 1.0 • 44.20 •278 • 1098.40 •0.00041560 • 98.76 • 100 • 190 • 1.90 x 103 • 1063 • Hint: Write numbers in scientific notation to help determine if leading zeros are significant. |
Significant Figures in Calculations • Don’t round for sig. figs. until the END of all calculations. • Multiplication and division: report to the least number of significant figures. = 12.61092 → 13 • Addition and subtraction: report to the least number of decimal places. = 1.977 → 1.98 |
| Sig Figs in Calculations
• Multiplication |
Significant Figures Practice • Calculate the following: • 1.67890 x 56.32 • 9.0210 + 856.1 • (6.02 + 1.5) x (3.14 + 2.579) |
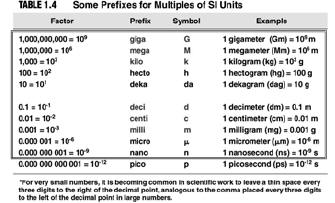
| Experimentation and Measurement • Metric prefixes
|
Dimensional Analysis • Allows us to convert from one unit to another • 1 dozen eggs = 12 eggs
• 1 inch = 2.54 cm
• 3 feet = 1 yard
|
| Metric Conversions • How many m are in 756 nm? • How many kg are in 12.34 g? • How many mL are in 1.450 L? • How many g are in 1907.12 mg? • How many mm are in 1.903 x 1010 m? |
Dimensional Analysis • A piece of string measures 5.5 inches long. How long is the string in mm? • 1 inch = 2.54 cm (will be given) •100 cm = 1 m (you need to know) • 1000 mm = 1 m (you need to know) |
| Dimensional Analysis Practice • Problem 1.17: Gemstones are weighed in carats, with 1 carat = 200 mg (exactly). What is the mass, in grams, of the Hope Diamond, the world’s largest blue diamond at 44.4 carats? • The density of a steel ball bearing is 7.81 g/cm3. If the ball bearing is measured to have a volume of 1.34 cm3, what is its mass in milligrams? |